Translate this page into:
Nail-fold capillaroscopy as a rapid non-invasive tool for assessment of microvascular complications in diabetes mellitus: A cross-sectional study
*Corresponding author: Dr. Prakhar Srivastava, Department of Dermatology and STD, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India. doctorprakharsjh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Srivastava P, Khunger N, Duvesh RK, Bansal S, Srivastava P. Nail-fold capillaroscopy as a rapid non-invasive tool for assessment of microvascular complications in diabetes mellitus: A cross-sectional study. J Onychol Nail Surg. 2024;1:4-12. doi: 10.25259/JONS_7_2024
Abstract
Background:
Chronic hyperglycaemia in diabetes leads to micro-vascular complications, like diabetic retinopathy, peripheral neuropathy and diabetic nephropathy. Microangiopathic changes can be detected and monitored in the nail fold capillaries using nail fold capillaroscopy.
Objectives:
The present study was undertaken to detect nailfold capillary changes in patients with diabetes mellitus by hand-held dermoscope and to find their association with disease duration, glycated haemoglobin (HbA1c) levels, and microvascular complications including retinopathy, nephropathy and peripheral neuropathy.
Material and Methods:
A cross-sectional study was conducted over 18 months on 100 diabetics and 100 controls. Screening for retinopathy, nephropathy and neuropathy was done. Nail-fold capillaroscopy (NFC) was done, and findings were graded according to the pre-defined criteria.
Results:
Out of the total 200 subjects included in the study, 100 were cases of diabetes, and 100 were controls. Twenty-five (25%) of participants with diabetes had Type 1 diabetes mellitus (T1DM), and 75 (75%) had Type 2 diabetes mellitus (T2DM). Overall, 26 (26%) of the apparently healthy controls were found to be pre-diabetic. NFC changes were present in 66 (66%) of Cases as opposed to 38 (38%) Controls. The mean NFC score was highest in T1DM (3.12), followed by T2DM (2.05), pre-DM (0.69) and controls (0.59). The NFC changes demonstrated an association with disease duration, glycaemic control, and micro-vascular complications.
Conclusion:
NFC is a simple, fast, inexpensive, non-invasive, and effective modality to analyse the functional and morphological details of the micro-vasculature. It could help in early diagnosis and timely intervention to prevent serious complications such as blindness, amputations, and renal failure in diabetics. NFC alterations were also observed in apparently healthy pre-diabetics. The small sample size of the study is one of the limitations
Keywords
Diabetes mellitus
Diabetes screening
Nail-fold capillaroscopy
Onychoscopy
INTRODUCTION
Diabetes mellitus (DM) encompasses a collection of metabolic disorders characterised by elevated blood glucose levels stemming from impaired insulin production, effectiveness, or a combination of both.[1] India is commonly referred to as the ‘Diabetes Capital of the World.’ In 2009, the worldwide prevalence of diabetes was estimated at 9.3% (463 million), with projections indicating a rise to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045.[2] The ‘Asia paradox’ highlights the swift socioeconomic and demographic transitions in the Asian population, positioning India as a focal point for the diabetes epidemic, with 69.2 million individuals affected. Annually, India sees three new cases of Type 1 diabetes per 100,000 children aged 0–14 years.[2]
Diabetes is a major contributor towards cardiovascular disease, blindness, kidney failure and lower limb amputation. Diabetic retinopathy, the most common microvascular complication, accounts for over 10,000 new cases of blindness each year. This condition may begin to develop as early as 7 years before a clinical diagnosis of Type 2 diabetes.[3]
Nail-fold capillaroscopy (NFC) is an emerging imaging technique used to examine nail-fold capillaries in both dermatology and rheumatology. In healthy individuals, NFC typically reveals hairpin-shaped capillaries arranged uniformly in a pattern resembling a comb, at the proximal nail fold.[4]
Chronic high blood sugar levels in diabetics lead to microvascular damage through mechanisms such as the presence of advanced glycation end products and the apoptosis of capillary pericytes and endothelial cells.[5] NFC can identify microvascular abnormalities in individuals with diabetes, including capillary tortuosity, irregular capillaries, capillary retraction, neo-angiogenesis and angulated capillaries. This technique is non-invasive, quick, reproducible, and provides a reliable qualitative and quantitative evaluation of the peripheral microvasculature in diabetic patients.[6]
While there is substantial research on diabetic microvascular changes in the retina, little is known regarding these changes in NFC. This study aims to describe NFC features in diabetic patients, with or without pre-existing microvascular complications, so as to understand its potential role in disease detection and monitoring.
MATERIAL AND METHODS
A cross-sectional study, under the Departments of Dermatology and Ophthalmology, was carried out over 18 months at a tertiary care hospital in Delhi. This study was initiated following institutional ethical clearance. The study included 100 participants, comprising both Type 1 (T1DM) and Type 2 DM (T2DM) patients, along with 100 age- and gender-matched controls. T1DM and T2DM were defined according to the criteria set by the American Diabetes Association. Patients with conditions affecting nail-fold microvasculature, such as hypertension, Raynaud’s phenomenon, collagen vascular disease, drug use (e.g., glucocorticoids and oral contraceptives), smoking, pregnancy, lactation, nail infections, onychophagia, onychotillomania, or occupations involving nail-fold trauma (e.g., farmers, gardeners), as well as those with ocular or retinal disease complicating fundus examination, were excluded from the study.
Diabetic nephropathy was defined by the presence of persistent proteinuria exceeding 500 mg of protein or 300 mg of albumin/24 hours, while excluding any urinary tract infections or other causes of proteinuria.[7] It was classified into three stages: Normal albuminuria (albumin-to-creatinine ratio <30 mg/mg), microalbuminuria (30:300 mg/mg) and macroalbuminuria (≥300 mg/mg).[8] Retinopathy was graded into no apparent retinopathy, non-proliferative diabetic retinopathy (further classified as mild, moderate and severe), and proliferative diabetic retinopathy.[9] Peripheral neuropathy was assessed using the Michigan Diabetic Neuropathy Score, which involved evaluating the appearance of the feet, presence of ulcers, assessment of ankle reflexes, along with sensory responses to vibration, light touch, pinpricks and monofilaments.[10]
NFC was performed after resting the subject for 15–20 min at ambient temperature (20–22°C). It was done with hands positioned on tabletop, at the level of the heart. All nail folds were examined, with the 4th and 5th fingers providing the most accurate results due to their skin transparency and minimal exposure to daily trauma. NFC was conducted using the DermLite DL3N dermatoscope, with both dry and wet contact dermatoscopy (using ultrasound jelly) employed. NFC alterations were graded according to the Hsu et al. criteria [Table 1],[11] and findings were documented in pre-designed proforma. Changes observed in at least two nail folds were considered significant. The results were statistically analysed for assessing significant correlations.[12]
| S. No. | Finding/Score | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|
| 1. | Capillary length | 200–500 | >500 | <200 | Variable |
| 2. | Capillary Distribution | Ordered | Comma-like | Irregular | Severe derangement |
| 3. | Capillary density | 10–30/mm2 | 8–10/mm2 | <8/mm2 | Severe alteration |
| 4. | Capillary diameter | Normal (8–14 mm) | Enlarged loop (>20 mm) | Giant loop (>50 mm) | - |
| 5. | Haemorrhages | Absent | In few areas | Diffuse | - |
| 6. | Subvenous plexus | Visible | Partially visible | Not appreciable | - |
| 7. | Flux | Normal | Slow | Discontinuous | Sludge phenomenon |
| 8. | Oedema/exudates | Absent | In few areas | Diffuse | - |
| 9. | Fundus | Transparent | Reduced transparence in few areas | Reduced transparence in all areas | - |
NFC: Nail-fold capillaroscopy
RESULTS
The mean age of the study subjects was 45.4 years. Among Cases, 25 (25%) had T1DM and 75 (75%) had T2DM. Thirty-five (35%) of the cases were female, while 65 (65%) were male. Twenty-six (26.0%) of the Controls were also found to be pre-diabetic as per the ADA criteria.[1] Among the controls, 37 (37%) were female, while 63 (63%) were male [Table 2]. The mean age of T1DM cases was 24.1 years, T2DM was 52.0 years, pre-DM was 40.6 years and healthy controls (n = 74) was 47.6 years. A family history of diabetes was present in more T2DM (29%) cases than T1DM (20%) cases. It was present in 34.6% of cases of pre-DM and 21.6% of healthy subjects.
| Parameters | Study Groups | P-value | |||
|---|---|---|---|---|---|
| Type 1 DM (n = 25) (%) |
Type 2 DM (n = 75) (%) |
Pre-Diabetes (n = 26) (%) |
Controls (n = 74) (%) |
||
| Age (Years) | 24.16±5.77 | 52.07±12.26 | 40.65±12.54 | 47.61±14.77 | |
| Gender | |||||
| Male | 8 (32.0) | 57 (76.0) | 14 (53.8) | 49 (66.2) | |
| Female | 17 (68.0) | 18 (24.0) | 12 (46.2) | 25 (33.8) | |
| Duration of disease | 6.60±4.49 | 6.73±6.18 | - | - | |
| Reduced capillary length | 3 (12.0) | 13 (17.3) | 0 | 0 | <0.001 |
| Irregular capillary distribution | 16 (64.0) | 20 (26.7) | 5 (19.2) | 15 (20.3) | <0.001 |
| Reduced capillary density | 1 (4.0) | 7 (9.3) | 0 | 0 | 0.015 |
| Dilated capillary loops | 15 (60.0) | 37 (49.3) | 5 (19.2) | 18 (24.3) | <0.001 |
| Presence of haemorrhages | 7 (28.0) | 9 (12.0) | 3 (11.5) | 0 | <0.001 |
| Presence of abnormal flux | 4 (16.0) | 2 (2.7) | 0 | 0 | 0.003 |
| Presence of oedema/exudates | 2 (8.0) | 2 (2.7) | 0 | 0 | 0.077 |
| Presence of tortuous capillaries | 10 (40.0) | 26 (34.7) | 9 (34.6) | 24 (32.4) | 0.924 |
| Neo-angiogenesis | 13 (52.0) | 16 (21.3) | 0 | 0 | <0.001 |
| Retinopathy | 9 (36.0) | 30 (40.0) | 0 | 0 | 0.723 |
| Neuropathy | 0 | 27 (36.0) | 0 | 0 | <0.001 |
| Nephropathy | 3 (12.0) | 14 (18.7) | 0 | 0 | 0.550 |
| Microvascular complications | 11 (44.0) | 37 (49.3) | 0 | 0 | 0.644 |
DM: Diabetes mellitus
Evidence of microvascular complications was present in 48% cases [diabetic retinopathy (39%), peripheral neuropathy (27%) and/or diabetic nephropathy (17%)]. Retinopathy was the most common complication in both T1DM and T2DM, being seen in 9 (36%) and 30 (40%) cases, respectively. Neuropathy was present in 27 (36%) cases of T2DM and absent in T1DM. Nephropathy was present in 3 (12%) cases of T1DM and 14 (18.7%) cases of T2DM. No microvascular complications were seen in pre-diabetics.
The mean NFC scores of different study groups is shown in Figure 1. NFC changes were present in 66 (66%) cases as opposed to 38 (38%) controls. The mean NFC score was highest in T1DM (3.12), followed by T2DM (2.05), pre-DM (0.69) and controls (0.59). Several NFC changes were seen in the study groups [Figure 2]. The NFC changes displayed a significant correlation with the duration of diabetes [Figure 3]. NFC alterations seen in healthy controls (n = 74) included tortuous capillaries in 24 (32.4%), dilated capillaries in 18 (24.3%) and irregular capillary distribution in 15 (20.3%) cases [Figure 4a and b]. NFC findings most commonly seen in pre-diabetics (n = 26) were tortuous capillaries (9, 34.6%) patients, irregular capillary distribution (5, 19.2%), dilated capillary loops (5, 19.2%) and focal haemorrhages (3, 11.5%) patients. Focal haemorrhage on NFC was the only change significantly associated with pre-DM as compared to healthy controls [Figure 5a and b].

- Graph depicting the mean of nail-fold capillaroscopy scores in the 4 study groups. NFC: Nailfold capillaroscopy, DM: Diabetes mellitus
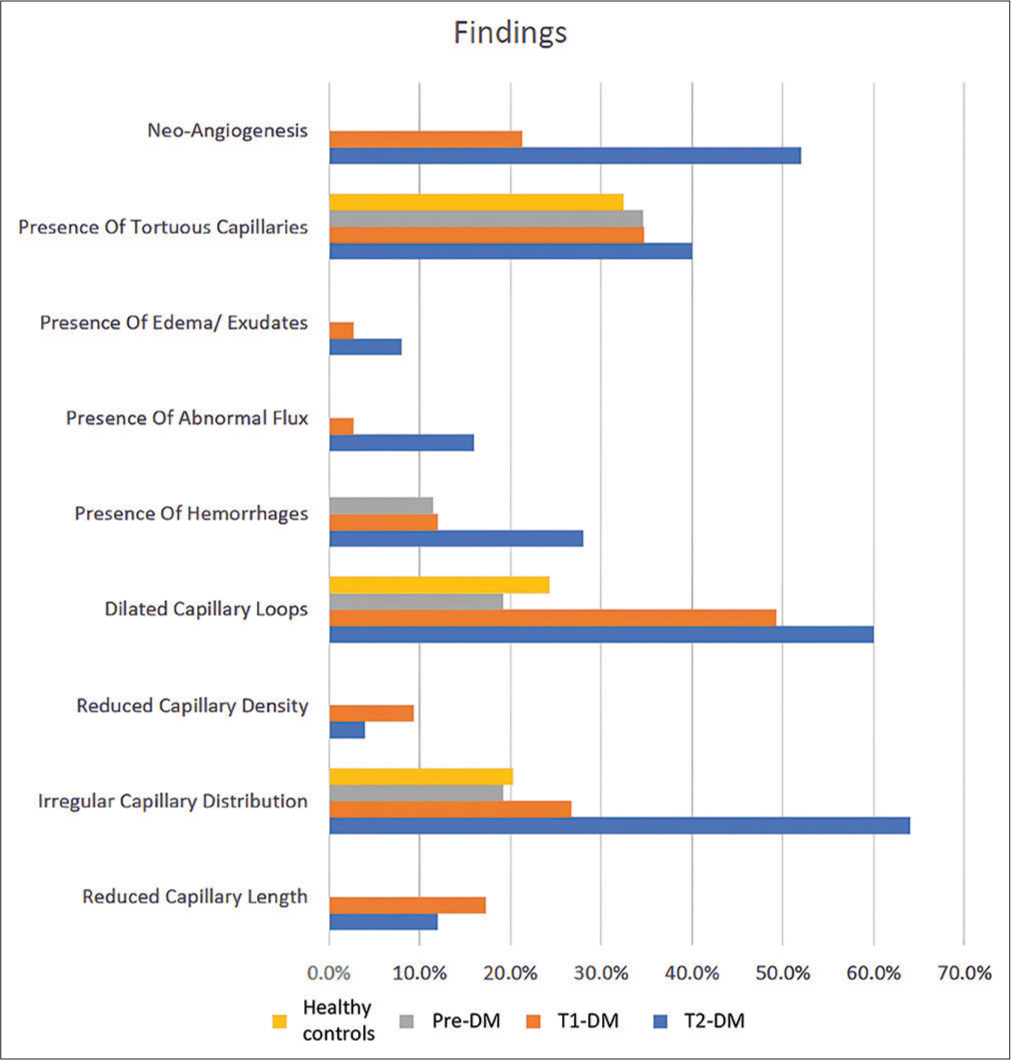
- Graph showing nailfold capillaroscopy changes in different study groups. T1DM: Type 1 diabetes mellitus, T2DM: Type 2 diabetes mellitus, DM: Diabetes mellitus

- Scatterplot depicting the correlation between nail-fold capillaroscopy score and duration of disease (Individual points represent individual cases. The blue trendline represents the general trend of correlation between the two variables. The shaded grey area represents the 95% confidence interval of this trendline). NFC: Nail-fold capillaroscopy

- (a) Nail-fold capillaroscopy (NFC) showing normal parallelly arranged hairpin vessels (white arrows) in a healthy control (b) NFC showing normal subpapillary capillary plexus (black arrow) in a healthy control (contact, polarised, DermLite DL3N, ×10).

- (a) Nail-fold capillaroscopy (NFC) showing tortuous and dilated capillary loops (black arrow) and capillary drop-outs (yellow arrow) in pre-diabetes mellitus (DM) (b) NFC showing tortuous and dilated capillary loops (black arrow) in pre-DM (contact, polarised, DermLite DL3N, ×10).
The most common NFC changes in diabetic cases (n = 100) were dilated capillaries (52%), tortuous capillaries (36%), irregular capillary distribution (36%), neo-angiogenesis (29%), reduced capillary length and haemorrhages (16% each). Most common NFC changes observed in T1DM (n = 25) was irregular capillary distribution (12, 48%). Other changes significantly associated with T1DM were bushy capillaries (11, 44%), dilated capillary loops (9, 36%), giant capillaries (6, 24%), focal haemorrhages (4, 16%), reduced capillary density (4, 16%) and reduced capillary length (3, 12%) patients [Figure 6]. The most common NFC change in T2DM (n = 75) was dilated capillaries (29, 38.7% patients). Other NFC findings significantly associated with T2DM included reduced capillary length (12, 16%), meandering capillaries (11, 14.7%), bushy capillaries (10, 13.3%), irregular capillary distribution (10, 13.3%), focal haemorrhages (9, 12%) and giant capillaries (8, 10.7%) [Figure 7a and b]. The NFC findings significantly associated with T1DM were irregular capillary distribution and bushy capillary as compared to T2DM.

- Nail-fold capillaroscopy showing tortuous capillary loop (red arrow) and bushy or meandering capillary loop (black arrow) in type 1 diabetes mellitus (contact, polarised, DermLite DL3N, ×10).

- (a) Nail-fold capillaroscopy (NFC) showing micro-haemorrhages (black arrow) and capillary drop-outs (red arrow) in uncontrolled type 2 diabetes mellitus (T2DM) (b) NFC showing micro-haemorrhages (red arrow), bushy capillary (black arrow) and giant capillaries (blue arrow) in T2DM with proliferative diabetic retinopathy (contact, polarised, DermLite DL3N, ×10).
The association between NFC alterations and microvascular complications of diabetes found NFC changes like dilated capillaries (70%), tortuous capillaries (58.3%), irregular capillary distribution (50%), neo-angiogenesis (43.8%) and reduced capillary length (31.2%) to be significantly associated. Even in cases where microvascular complications of diabetes were not established clinically, NFC showed early microvascular changes in the form of dilated capillaries (34.6%), irregular capillary distribution (23.1%), tortuous capillaries (15.4%) and neo-angiogenesis (15.4%). The mean NFC score was significantly higher in DM with retinopathy (4.05) than in DM without retinopathy (1.21). The NFC changes significantly associated (P < 0.05) with diabetic retinopathy were dilated capillaries (31, 79.5%), tortuous capillaries (25, 64.1%), irregular capillary distribution (22, 56.4%), neo-angiogenesis (20, 51.3%), reduced capillary length (13, 33.3%) haemorrhages (10, 25.6%) and reduced capillary density (7, 17.9%). NFC changes significantly associated (P < 0.05) with diabetic neuropathy were dilated capillaries (20, 74.1%), tortuous capillaries (14, 51.9%), irregular capillary distribution (14, 51.9%), reduced capillary length (10, 37%) and reduced capillary density (5, 18.5%). Stage-1 nephropathy was present in 16 (16%) and Stage-2 nephropathy in 1 (1%) of diabetes patients. Nephropathy was significantly associated (P < 0.05%) with tortuous capillaries (13, 76.4%), reduced capillary length (12, 70.5%), abnormal capillary distribution (11, 64.7%), dilated capillaries (10, 58.8%), bushy capillaries (7, 41.1%), meandering capillaries (6, 35.3%), partially visible subvenous plexus (6, 35.3%), reduced capillary density (4, 23.5%) and giant capillaries (4, 23.5%) patients. Neoangiogenesis was specifically associated with retinopathy and nephropathy, while haemorrhage and abnormal flux were significantly associated with retinopathy.
NFC changes showed significant association with a deranged haemoglobin A1c (HbA1c) levels (>5.6%). Changes significantly associated were reduced capillary length, reduced capillary density, dilated capillary loops, micro-haemorrhage, and evidence of neo-angiogenesis (P < 0.05%). The mean NFC score (1.98) was significantly greater (P < 0.001) in patients with deranged HbA1c than in those with normal HbA1c (0.59). There was a statistically significant, strong positive correlation between NFC score and duration of disease (P < 0.001, rho=0.62). For every 1 year increase in duration of disease, the NFC score increased by 0.27 units [Figure 3].
DISCUSSION
As per current global statistics, 463 million individuals have diabetes. India accounts for 77 million of these.[13] The prevalence of T2DM among individuals aged 2079 in India stands at 8.9%.[12] According to the Centre for Cardiometabolic Risk Reduction in South Asia study, six out of every ten adults in South Asian cities have either diabetes or prediabetes. In Delhi, approximately 25.2% of the population is affected by diabetes.[14] India also bears the largest burden of T1DM in the Southeast Asian region, accounting for 23% of the global T1DM burden.[13] In our study, 26% of the 100 controls were found to be prediabetic. Globally, about 374 million people have impaired glucose tolerance, which is considered a prediabetic condition.[13] The Indian Council of Medical Research– India DIABetes study revealed prediabetes prevalence rates ranging from 5.8% to 14.7% in rural India and 7.2% to 16.2% in urban areas. Notably, the prevalence of prediabetes was higher than that of diabetes in most Indian states, indicating a large population at risk of developing T2DM in the near future.[15,16]
Dermoscopy serves as an intermediate tool between clinical dermatology (macroscopic) and histopathology (microscopic).[17] We used dermoscopy with ultrasound gel to perform wet contact NFC for both diabetic patients and healthy controls. Tasli suggested that ultrasound gel is the preferred medium for dermoscopy in nail examinations.[18] In our healthy controls, NFC revealed tortuous capillaries in 24 (32.4%), dilated capillaries in 18 (24.3%) and irregular capillary distribution in 15 (20.3%) cases. Jakhar et al. reported similar findings in healthy individuals with tortuous capillaries (22%), dilated capillaries (6%), bushy capillaries (4%) and bizarre capillaries (2%).[4] However, bushy, meandering, bizarre capillaries or capillary haemorrhages were not observed in our control population. This is in alignment with the findings by Ingegnoli et al., who also found these to be uncommon in healthy subjects.[19] Tortuosity was the most frequent NFC change in healthy subjects in several other studies as well.[4] Ríos et al. and Hoerth et al. documented capillary changes, such as tortuous, cross-linked and ramified capillaries, in healthy individuals.[20,21]
In prediabetic patients, NFC changes included tortuous capillaries (34.6%), irregular capillary distribution (19.2%), dilated capillary loops (19.2%) and focal haemorrhages (11.5%). Focal haemorrhages were the only change significantly associated with prediabetes as compared to healthy controls. The mean NFC score in our study was 0.69 as compared to a score of 1 in the study by Hsu et al.[11]
Capillary changes are more frequently observed in diabetics with poor metabolic control and longer disease duration. In our study, dilated capillary loops were observed with a mean diabetes duration of 9.47 years and giant capillary loops with a mean duration of 9.93 years. Dilated capillaries were seen in 36% cases of T1DM, 38.7% of T2DM, 24.3% of prediabetics and 30.5% healthy controls. Similarly, giant capillaries were present in 24% cases of T1DM and 10.7% of T2DM, and were significantly associated with both conditions as compared to prediabetics and healthy controls (P < 0.05). Ectatic and giant capillaries signify early stages of peripheral microangiopathy, signalling an early abnormality in blood flow and should be considered ‘red flags’ for undetected microangiopathy.[22] Focal haemorrhages were observed after an average disease duration of 9.93 years, while diffuse haemorrhages appeared after 14 years. Micro-haemorrhages were found in 24% T1DM cases, 12% T2DM cases, 11.5% prediabetics, and absent in healthy controls. They were strongly associated with both T1DM (P = 0.001) and T2DM (P = 0.001) as compared to healthy controls. Focal haemorrhages were the only NFC change significantly correlated with prediabetes. Micro-haemorrhages result from capillary loop damage and are an early indicator of microvascular disease, acting as a ‘bridge’ between giant capillaries and avascular areas.[23]
Tissue hypoxia, a result of vessel abnormalities and impaired blood flow, triggers the release of vascular endothelial growth factor, promoting formation of abnormal new vessels, known as neo-angiogenesis. These appear on NFC as meandering, bushy or bizarre capillaries.[23] Neo-angiogenesis was significantly associated with both T1DM and T2DM.
Reduced capillary density, which was observed with a mean diabetes duration of 13.38 years, occurs in long-standing uncontrolled diabetes and is a predictor of poor prognosis, signalling irreversible microangiopathy.[23] In our study, 8% diabetics showed reduced capillary density. Cicco also found a 28% decrease in capillary density among diabetic patients.[24] In T1DM, NFC changes included irregular capillary distribution in 12 (48%) cases, bushy capillaries in 11 (44%), dilated capillaries in 9 (36%), giant capillaries in 6 (24%), focal haemorrhages in 4 (16%), reduced capillary density in 4 (16%) and shortened capillary length in 3 (12%). Kuriliszyn-Moskal et al. similarly reported dilated capillaries, tortuosities and reduced capillary density in T1DM patients as compared to controls.[25] Hosking et al. found that micro-haemorrhages and avascular areas were the most common NFC changes in T1DM and reported that patients with microvascular complications had more avascular areas.[26]
The most common NFC change in T2DM was the presence of dilated capillary loops (38.7%). Other NFC changes significantly associated with T2DM (P = 0.05) compared to healthy controls included reduced capillary length (16%), meandering capillaries (14.7%), bushy capillaries (13.3%), irregular capillary distribution (13.3%), focal haemorrhages (12%) and giant capillaries (10.7%). T2DM patients exhibited more tortuous and dilated capillaries than healthy individuals.[12] Nodular apical elongations have been linked to longer disease duration.[12] In addition, uniquely altered capillaries, including ‘angulated’ and ‘receding’ capillaries, have been described in T2DM.[27] In our study, 9.3% of T2DM cases exhibited reduced capillary density. Chang et al. highlighted regressional changes, rather than proliferative ones, in NFC among people with diabetes.[28]
We found that irregular capillary distribution and bushy capillaries were the two significant NFC findings distinguishing T1DM from T2DM. The mean NFC score was higher in T1DM (3.12) compared to T2DM (2.05), indicating more pronounced NFC changes in T1DM. Barchetta et al. similarly reported that T1DM patients exhibited more capillaroscopic changes than those with T2DM.[29]
Our study identified NFC changes in diabetics even in the absence of clinically established microvascular complications. The mean NFC score for diabetics without microvascular complications was 1.12. Barchetta et al. also noted capillaroscopic alterations in nearly half of patients without retinopathy, suggesting early microangiopathic changes.[29] NFC changes significantly associated with diabetic microvascular complications in our study included dilated capillaries (70%), tortuous capillaries (58.3%), irregular capillary distribution (50%), neo-angiogenesis (43.8%) and reduced capillary length (31.2%). Jakhar et al. also reported more morphological changes in diabetics with microvascular complications.[27] Neo-angiogenesis, haemorrhages and abnormal capillary flux were specific findings significantly associated with retinopathy. Bakirci et al. also noted that T2DM patients with diabetic retinopathy had more frequent capillary haemorrhages, ectasia, giant capillaries and neoangiogenesis, although these findings were not statistically significant.[30] Chang et al. found greater tortuosity in patients with retinopathy.[28] Uyar et al. and Chang et al. also reported an increased prevalence of tortuous, bushy and dilated capillaries in diabetics with retinopathy.[28,31] Similar to our results, Hsu et al. demonstrated a positive correlation between NFC scores and both diabetic neuropathy and the number of microvascular complications.[11] We also found a significant association between NFC changes and nephropathy. However, Hsu et al. did not find a similar association with nephropathy in their study.[11]
In our research, NFC changes were significantly associated with the overall duration of diabetes, with each one year increase in disease duration corresponding to a 0.27-unit increase in the NFC score. Kuryliszyn et al. and Bollinger et al. similarly observed more severe NFC changes with longer disease duration.[25,32] In contrast, Barchetta et al. and Pazos-Moura et al. found no such correlation.[22,29]
We found that patients with higher HbA1c levels had a significantly greater mean NFC score (1.98) as compared to those with normal HbA1c (0.59) (0.001). Jakhar et al. did not find any significant correlation between NFC parameters and glycaemic control levels.[27] Kaminska-Winciorek et al. and Barchetta et al. concluded that microvascular changes in people with diabetes were not influenced by age or sex.[29,33]
Limitations
The small sample size is one of the limitations. Further studies could be undertaken with follow-up of cases for the development of microvascular complications. Furthermore, pre-DM is a cohort that needs more scientific exploration so that effective screening guidelines can be formulated.
CONCLUSION
NFC changes in diabetics showed an association with disease duration, glycaemic control, and the presence of micro-vascular complications. This could help in early diagnosis and timely intervention to prevent serious complications such as blindness, amputations, and renal failure. NFC alterations were also observed in apparently healthy pre-diabetics, most of whom had a positive family history. Therefore, we recommend NFC as a simple, useful bedside assessment tool to screen for micro-vascular complications in diabetes and pre-diabetes.
Authors’ contributions
All the authors’ contributed to the design, literature search, clinical studies, concepts, experimental studies, data acquisition, data analysis, statistical analysis and manuscript editing, preparation and reviewing.
Ethical approval
The research was approved by the Institutional Review Board at Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, number IEC/VMMC/SJH/Thesis/2020-11/CC-87, dated December 10, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Niti Khunger and Dr. Shikha Bansal are on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Classification and diagnosis of diabetes: Standards of Medical Care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13-28.
- [CrossRef] [PubMed] [Google Scholar]
- Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas In: Diabetes Res Clin Pract Vol 157. (9th edition). 2019. p. :107843.
- [CrossRef] [PubMed] [Google Scholar]
- Preventive care in rural primary care practice. Cancer. 1993;72(3 Suppl):1113-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nailfold capillaroscopy with USB dermatoscope: A cross-sectional study in healthy adults. Indian J Dermatol Venereol Leprol. 2020;86:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab. 2014;3:94-108.
- [CrossRef] [PubMed] [Google Scholar]
- Nailfold capillaroscopy in diabetes mellitus. Microvasc Res. 2017;112:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254-64.
- [CrossRef] [PubMed] [Google Scholar]
- Microalbuminuria in type 2 diabetes and hypertension: A marker, treatment target, or innocent bystander? Diabetes Care. 2008;31(Suppl 2):S194-201.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-4.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab. 2016;31:230-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nailfold capillary abnormalities are associated with type 2 diabetes progression and correlated with peripheral neuropathy. Medicine (Baltimore). 2016;95:e5714.
- [CrossRef] [PubMed] [Google Scholar]
- Nail-fold capillaroscopy for the dermatologists. Indian J Dermatol Venereol Leprol. 2022;88:300-12.
- [CrossRef] [PubMed] [Google Scholar]
- IDF Diabetes Atlas In: Brussels (8th and 9th ed). Belgium: International Diabetes Federation; 2019.
- [Google Scholar]
- High burden of prediabetes and diabetes in three large cities in South Asia: The Center for cArdio-metabolic risk reduction in South Asia (CARRS) study. Diabetes Res Clin Pract. 2015;110:172-82.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585-96.
- [CrossRef] [PubMed] [Google Scholar]
- Type 2 diabetes in migrant south Asians: Mechanisms, mitigation, and management. Lancet Diabetes Endocrinol. 2015;3:1004-16.
- [CrossRef] [PubMed] [Google Scholar]
- Onychoscopy: A practical guide. Indian J Dermatol Venereol Leprol. 2017;83:536-49.
- [CrossRef] [PubMed] [Google Scholar]
- The role of various immersion liquids at digital dermoscopy in structural analysis. Indian J Dermatol Venereol Leprol. 2011;77:110.
- [CrossRef] [PubMed] [Google Scholar]
- Nailfold capillary patterns in healthy subjects: A real issue in capillaroscopy. Microvasc Res. 2013;90:90-5.
- [CrossRef] [PubMed] [Google Scholar]
- Study of capillary patterns in healthy population. Rev Arg Reum. 2016;27:27-31.
- [CrossRef] [Google Scholar]
- Qualitative and quantitative assessment of nailfold capillaries by capillaroscopy in healthy volunteers. Vasa. 2012;41:19-26.
- [CrossRef] [PubMed] [Google Scholar]
- Nailfold capillaroscopy in diabetes mellitus: Morphological abnormalities and relationship with microangiopathy. Braz J Med Biol Res. 1987;20:777-80.
- [Google Scholar]
- Nailfold capillaroscopy: A comprehensive review on common findings and clinical usefulness in non-rheumatic disease. J Med Invest. 2021;68:6-14.
- [CrossRef] [PubMed] [Google Scholar]
- Hemorheology and microcirculation in some pathologies of internal medicine. Minerva Med. 2007;98:625-31.
- [Google Scholar]
- Microvascular abnormalities in capillaroscopy correlate with higher serum IL-18 and sE-selectin levels in patients with type 1 diabetes complicated by microangiopathy. Folia Histochem Cytobiol. 2011;49:104-10.
- [CrossRef] [PubMed] [Google Scholar]
- Non-invasive detection of microvascular changes in a paediatric and adolescent population with type 1 diabetes: A pilot cross-sectional study. BMC Endocr Disord. 2013;13:41.
- [CrossRef] [PubMed] [Google Scholar]
- Nail fold capillaroscopic changes in patients with type 2 diabetes mellitus: An observational, comparative study. Indian J Med Spec. 2020;11:28-33.
- [CrossRef] [Google Scholar]
- Use of dynamic capillaroscopy for studying cutaneous microcirculation in patients with diabetes mellitus. Microvasc Res. 1997;53:121-7.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy: Capillary alterations in patients with diabetes. Diabet Med. 2011;28:1039-44.
- [CrossRef] [PubMed] [Google Scholar]
- The evaluation of nailfold videocapillaroscopy findings in patients with type 2 diabetes with and without diabetic retinopathy. North Clin Istanb. 2019;6:146-50.
- [Google Scholar]
- Assessment of the relationship between diabetic retinopathy and nailfold capillaries in type 2 diabetics with a noninvasive method: Nailfold videocapillaroscopy. J Diabetes Res. 2016;2016:7592402.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical capillaroscopy: A guide to its use in clinical research and practice Boston, MA: Hogrefe and Huber Publishing; 1990.
- [Google Scholar]
- Diabetic microangiopathy in capillaroscopic examination of juveniles with diabetes type 1. Post Hig Med Dosw. 2012;66:51-9.
- [CrossRef] [PubMed] [Google Scholar]








